|
| |
Get 15 % off on a Livescribe
Smartpen and help us maintain this site!
Instructions:
| This application can recognize 150 2D and 239 3D
acyclic alkane structures with up to 10 carbons or 9 lines! The
highlighted text is the pen's display.
Once you have entered
Acyclic Alkanes, you can select one of three options
from the vertical navigation menu:
| Option |
What you can
do? |
|
Tutorial
Mode: |
See the name of your drawing. |
|
Quiz Mode: |
Draw a given acyclic alkane. |
|
Instructions Website: |
Remember where to find instructions. |
|
|
Tutorial
Mode: Once you see
Draw
displayed, draw a straight line that is longer than the
separation of two lines on college rule paper: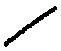 .
The display shows
ethane. Lift up the pen and draw
another line connected to the first line: .
The display shows
ethane. Lift up the pen and draw
another line connected to the first line: .
The display shows propane. Draw a third line branching from the center of the
first two lines: .
The display shows propane. Draw a third line branching from the center of the
first two lines: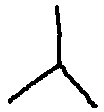 .
The display shows 2-methylpropane. You can start a new drawing by reentering
the Tutorial
Mode:, by double tapping on the paper twice or by navigating
right. .
The display shows 2-methylpropane. You can start a new drawing by reentering
the Tutorial
Mode:, by double tapping on the paper twice or by navigating
right. |
|
Quiz Mode: You will see
a display like
Draw (R)-2,3-dimethylpentane.
As you add each line you will see the name of the structure that you have drawn
followed by a comma, and a prompt to draw the same thing, e.g.,
2,3-dimethylpentane, Draw
(R)-2,3-dimethylpentane for
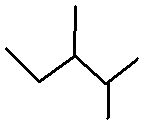 . Complete
a wedge by drawing (in one stroke) a figure that
looks like a 7 or an L between two points that already have a line between
them, e.g.,
Correct!: . Complete
a wedge by drawing (in one stroke) a figure that
looks like a 7 or an L between two points that already have a line between
them, e.g.,
Correct!: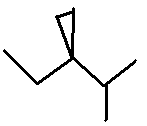 .
The wedge means that the atom at the end of the stroke is in front of the atom
at the beginning of the stroke. Double tap on the paper twice to try
drawing the
same question. .
The wedge means that the atom at the end of the stroke is in front of the atom
at the beginning of the stroke. Double tap on the paper twice to try
drawing the
same question. |
|
Messages*:
|
CcHh |
The molecular formula is displayed because the program does not
know the name of your drawing. |
|
No
bond? |
Your line was not straight, you drew a wedge between two points
that did not have a line between them or you drew an atomic symbol
before its bond. |
|
Please wait... |
If the recognition of your drawing is taking too long, exit the
program. |
|
Tap Twice to Erase! |
You double tapped once. You have to double tap again or
continue drawing. |
|
Unknown Element? |
You entered an atomic symbol besides C or H or you drew a bond that
was too short. |
*Different auditory clues are provided for
each operation. |
Background:
-
Acyclic alkanes contain only carbon
and hydrogen but do not have any rings or multiple bonds between the same
two atoms.
-
They are the first things you learn to name in
organic chemistry.
-
This application can recognize all 239
three-dimensional acyclic alkanes with up to 10 carbons.
-
The shorthand for drawing them is lines and
wedges.
-
Lines represent the bonds between atoms.
-
Wedges represent atoms sticking out of
the plane of the sheet of paper.
-
In shorthand drawings, where two lines meet or
the end of a line (in mathematics this point is called a vertex of a
graph) represents a carbon with an appropriate number of hydrogens.
-
The maximum number of lines that should be
drawn to a point representing a carbon is four and the number of
implicit hydrogens is four minus the number of lines meeting at the
point. For instance, if you draw one line you are representing two
carbons (the two ends of the line) plus six hydrogens (3 hydrogens
per end of the line).
-
If you use the atomic symbol C in your drawing
then you must also draw your own hydrogens using the atomic symbol
H.
-
By just using lines you can learn the name of
all acyclic alkanes with up to 10 carbons.
-
By using atomic symbols you can check that you
known how many carbons and hydrogens are represented in a shorthand
drawing. You must draw methane this way.
-
By using wedges you can enter the secret 3D world of
stereochemistry.
General Rules for Naming Acyclic Alkanes
Systematically:
-
Name the longest chain with the most branches a follows:
-
propane, 3
-
butane, 4
-
pentane, 5
-
hexane, 6
-
heptane, 7
-
octane, 8
-
nonane, 9
-
decane, 10
-
Name simple branches by length as follows:
-
methyl, 1
-
ethyl, 2
-
propyl, 3
-
More complicated branches are named as branches of branches
such as:
-
1-methylethyl
-
1-methylpropyl
-
2-methylpropyl
-
List the branches as prefixes in alphabetical order, e.g.,
ethylmethylpentane, with no spaces between the parts.
-
Number the branches as prefixes to give the smallest
numbering, e.g., 2-methylpentane not 4-methylpentane, separating numbers
from letters with hyphens.
-
Condense multiple listings of the same branch name with the
prefixes di, tri, tetra, penta, etc., e.g., 2,3-dimethylbutane, separating
number from each other with commas.
-
There are more rules.
-
Buy this
program and draw the figures below to learn to name acyclic alkanes
systematically.
General Rules for Naming Stereo Acyclic Alkanes:
|
Click below to see all the acyclic alkanes this program can name!
| |
|
 Acyclic Alkanes
Acyclic Alkanes